Researchers reprogram yeast cells to produce medium-chain fatty acids as palm oil alternative
German and Chinese researchers have developed a protein engineering approach that reprograms metazoan fatty acid synthase to produce short- and medium-chain fatty acids with unprecedented specificity. The technology, demonstrated in the industrial yeast Ogataea polymorpha, offers a sustainable alternative to palm kernel oil extraction for food.
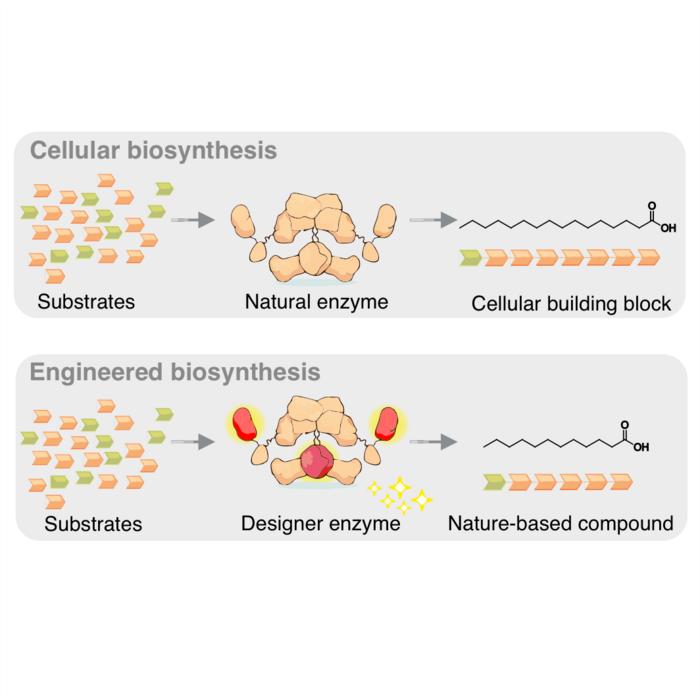
Schematic representation of biosynthesis in a cell (top) and in the laboratory (bottom). The designer enzyme shortens the chain length of the fatty acid. © Felix Lehmann & Martin Grininger/Goethe University
A collaborative research effort between Goethe University Frankfurt and the Dalian Institute of Chemical Physics has yielded a breakthrough in programmable fatty acid biosynthesis. The team engineered metazoan fatty acid synthase (mFAS) to fine-tune the interplay between fatty acid chain extension and hydrolytic release, enabling targeted production of industrially valuable short- and medium-chain fatty acids (SMCFAs).
The research, published in Nature Chemical Biology, addresses a critical challenge in oleochemical manufacturing. Medium-chain fatty acids, particularly lauric acid (C12-FA), are essential ingredients in surfactants, detergents, lubricants and personal care products. Currently, these compounds are predominantly sourced from palm kernel oil, which constitutes less than 10% of palm fruit and contains approximately 50% C12-FA, necessitating large-scale cultivation with associated environmental impacts.
Engineering the ketosynthase domain
The researchers identified that single amino acid exchanges in the ketosynthase (KS) domain could redirect fatty acid product profiles. The G113W mutation produced predominantly C8 fatty acids, whilst G113F yielded C8/C10 and G113M generated C12/C14 profiles. These modifications were combined with replacement of the native thioesterase domain with ‘TesA from Escherichia coli, creating what the team termed a “generalist mFAS” platform.
Prof. Martin Grininger of Goethe University Frankfurt explained the underlying principle: “FAS is one of the most important enzymes in a cell’s metabolism and has been fine-tuned by evolution over millions of years.” He added: “Fundamentally, our advantage lies in the very precise control of chain length. We can theoretically make any chain length, and we’re demonstrating this with the example of C12 fatty acid, which otherwise can only be obtained from palm kernels or coconut.”
Doctoral student Damian Ludig, who led the protein engineering work, described the approach: “Two changes to FAS through protein engineering ultimately led us to our goal. In the ketosynthase subunit, I first exchanged one amino acid which resulted in chains being extended only with low efficiency beyond a certain length. Additionally, I replaced the thioesterase subunit with a similar protein from bacteria that shows activity in cleaving short chains.”
Yeast cell factory development
The collaboration with Prof. Yongjin Zhou’s research group at the Dalian Institute of Chemical Physics translated the engineered enzymes into industrial yeast strains. Using the thermotolerant yeast Ogataea polymorpha, the team implemented a multi-faceted metabolic engineering strategy.
Key modifications included deletion of the fatty acid oxidase gene POX1, which increased MCFA titres threefold, and remodelling of the β-oxidation pathway to selectively degrade long-chain fatty acids whilst preserving medium-chain products. The endogenous acyl-CoA ligase OpFAA1 was replaced with Saccharomyces cerevisiae ScFAA1, which preferentially activates long-chain fatty acids, enabling MCFA accumulation.
The optimised strain XMCFA69 achieved 90.8 mg l⁻¹ MCFA production in shake flask cultures, with C12-FA comprising 69% of total free fatty acids. Fed-batch fermentation in 1.5-litre bioreactors yielded 708.6 mg l⁻¹ total MCFAs, consisting of 189.5 mg l⁻¹ C10-FA, 484.1 mg l⁻¹ C12-FA and 34.0 mg l⁻¹ C14-FA. The C12-FA specificity achieved is comparable to that found in palm kernel oil and coconut oil.
Implications for food manufacturing
The authors note that their work “demonstrates a modular platform for programmable FA synthesis and paves the way toward sustainable bioproduction of valuable oleochemicals.” They further note: “Given the industrial relevance of O. polymorpha, these findings underscore the strong potential of mFAS/’TesA hybrids for developing efficient cell factories for sustainable SMCFA production.”
The technology extends beyond fatty acids. Integration of a thioreductase domain enabled direct enzymatic synthesis of medium-chain fatty aldehydes and alcohols, expanding the product portfolio for flavour, fragrance and functional ingredient applications.
Both laboratories have filed patents for their technologies. Grininger noted: “On the Chinese side, Unilever was involved in this project. Our development has thus far taken place without industrial participation. However, we are striving for a collaboration with an industry partner in order to bring the technology into application.”
The research was supported by the German Research Foundation, the National Natural Science Foundation of China and Unilever. Successful scale-up alongside industry partners will determine whether this biotechnological approach can provide a viable alternative to tropical oil extraction for the food, cosmetic and personal care industries.
Reference
Ludig, D. L., Zhai, X., Rittner, A., et. al. (2026). Engineering metazoan fatty acid synthase to control chain length applied in yeast. Nature Chemical Biology. https://doi.org/10.1038/s41589-025-02105-w



